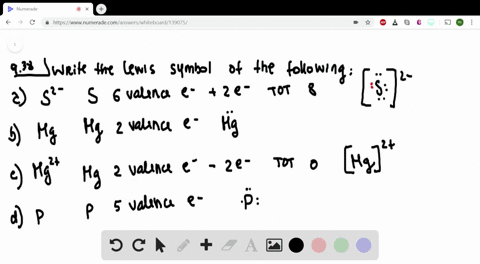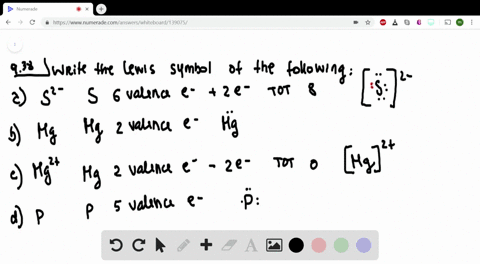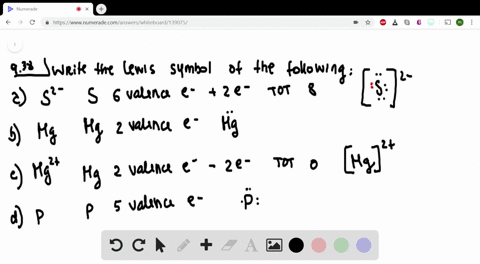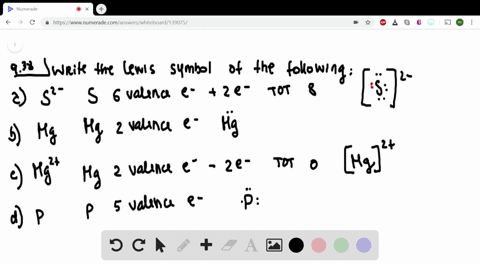
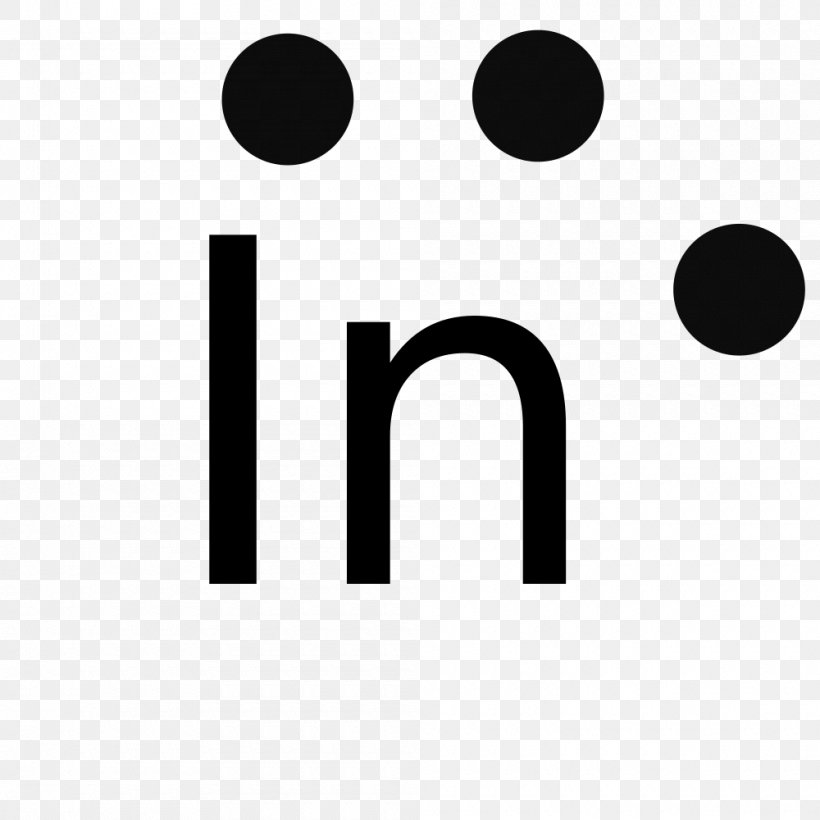
42 lewis symbols
Lewis Symbols - Chemistry LibreTexts A Lewis Symbol is constructed by placing dots representing electrons in the outer energy around the symbol for the element. For many common elements, the number of dots corresponds to the element's group number. Below are Lewis Symbols for various elements. Notice the correspondence to each element's group number. Lewis Symbols and Structures – Chemistry - UH Pressbooks We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: [link] shows the Lewis symbols for the elements of the third period of the periodic table. Lewis symbols illustrating the number of valence ...
Lewis Symbols and Structures | Chemistry | | Course Hero A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 1 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 1. Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table.
Lewis symbols
Share on Twitter A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 1 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 1. Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. Lewis symbols can ... What is the Lewis symbol of fluorine? These Lewis symbols and Lewis structures help visualize the valence electrons of atoms and molecules, whether they exist as lone pairs or within bonds. 37 Related Question Answers Found Is fluorine F or f2? Fluorine. Fluorine is the chemical element in the periodic table that has the symbol F and atomic number 9. Lewis Symbols and Structures - chem-textbook A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: shows the Lewis symbols for the elements of the third period of the periodic table. Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. Lewis symbols can also be used to ...
Lewis symbols. 4.4 Lewis Symbols and Structures - Chemistry: Atoms First | OpenStax We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 4.10 shows the Lewis symbols for the elements of the third period of the periodic table. 6.01 Lewis symbols - Week 6 | Coursera These structures provide information about the types of bonds (single, double, or triple) as well as the connectivity of atoms. By knowing the Lewis structure, we can also predict the three-dimensional geometry of an individual molecule. 6.01 Lewis symbols 4:36. 6.02 Covalent bonds 5:19. 6.03 Electronegativity 11:21. 7.3 Lewis Symbols and Structures - Chemistry | OpenStax A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 7.9 Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. Lewis Dot Symbols and Lewis Structures | Boundless Chemistry | | Course ... The Lewis symbol for carbon: Each of the four valence electrons is represented as a dot. Electrons that are not in the valence level are not shown in the Lewis symbol. The reason for this is that the chemical reactivity of an atom of the element is solely determined by the number of its valence electrons, and not its inner electrons. Lewis ...
Lewis Symbols - Chemical Bonding and Molecular Structure G.N. Lewis introduced simple symbols to denote the valence electrons in an atom. The outer shell electrons are shown as dots surrounding the symbol of the atom. These symbols are known as Lewis symbols are electron dot symbols. Significance. The number of dots around the symbol give the number of electrons present in the outermost shell. Lewis Symbols and Structures (4.4) - Chemistry 110 We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol. consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 4.9 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 4.9 Lewis symbols illustrating the number of ... 7.3 Lewis Symbols and Structures - Chemistry A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 1 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 1. Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. 4.4 Lewis Symbols and Structures - General Chemistry 1 & 2 Lewis Symbols. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 1 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 1. Lewis symbols illustrating the ...
7.3: Lewis Symbols and Structures - Chemistry LibreTexts A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.3.1 shows the Lewis symbols for the elements of the third period of the periodic table. Electron dots are typically arranged in four pairs located on the four "sides" of the atomic symbol. Lewis structure - Wikipedia Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures ... Lewis structures show each atom and its position in the structure of the molecule using its chemical symbol. Lines are drawn between atoms that are bonded to one another (pairs of dots can be used instead of ... 40. Lewis Symbols and Structures - BC Open Textbooks We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: (Figure) shows the Lewis symbols for the elements of the third period of the periodic table. Lewis symbols illustrating the number of valence ... Lewis Symbols and Structures - Introductory Chemistry Lewis Symbols. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: The table below shows the Lewis symbols for the elements of the third period of the periodic table. Lewis symbols illustrating the ...
Lewis Symbols or Diagrams - Elmhurst University A Lewis Symbol consists of the element symbol surrounded by "dots" to represent the number of electrons in the outer energy level as represented by a Bohr Diagram. The number of electrons in the outer energy level is correlated by simply reading the Group number. Lewis symbols for oxygen, fluorine, and sodium are given in the diagram on the left.
Lewis Symbols and the Octet Rule | Chemistry | JoVE The Lewis symbol for neon has eight dots, two dots on each side, representing the filled electron configuration; in other words, an octet. The octet rule states that an atom tends to lose, gain, or share electrons in the form of bonds until a stable electron configuration, an octet, is reached. Consider carbon dioxide.
6.1 Lewis Symbols and Structures - Chemistry Fundamentals We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 6.1.1 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 6.1.1 Lewis symbols illustrating the number ...
7.3 Lewis Symbols and Structures - Chemistry A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 1 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 1. Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. Lewis symbols can ...
Lewis Symbols | Chemical Bonding - Nigerian Scholars Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. Lewis symbols can also be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium: Likewise, they can be used to show the formation of anions from atoms, as shown here for chlorine and ...
Lewis Structure Definition and Example - ThoughtCo A Lewis structure is based on the concept of the octet rule, in which atoms share electrons so that each atom has eight electrons in its outer shell. As an example, an oxygen atom has six electrons in its outer shell. In a Lewis structure, these six dots are arranged so that an atom has two lone pairs and two single electrons.
Groß-Karben, Hesse, Germany Weather - The Weather Channel Latest COVID-19 coronavirus data and map for Groß-Karben, Hesse, Germany
Lewis symbols - ChemSimplified
Lewis Structures: Dot Symbols, Diagram, Examples - Embibe Lewis Structures: As valence electrons are significant to an atom's reactivity, it is essential to represent it by simple diagrams.Lewis Structures are pictorial representations of molecules in which the valence electrons present in an atom are represented as dots.
Lewis Symbols and Structures - chem-textbook A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: shows the Lewis symbols for the elements of the third period of the periodic table. Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. Lewis symbols can also be used to ...
What is the Lewis symbol of fluorine? These Lewis symbols and Lewis structures help visualize the valence electrons of atoms and molecules, whether they exist as lone pairs or within bonds. 37 Related Question Answers Found Is fluorine F or f2? Fluorine. Fluorine is the chemical element in the periodic table that has the symbol F and atomic number 9.
Share on Twitter A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 1 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 1. Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. Lewis symbols can ...
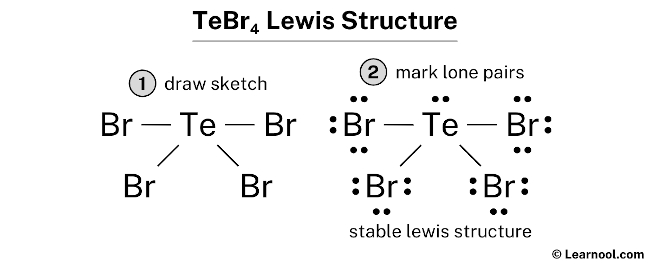





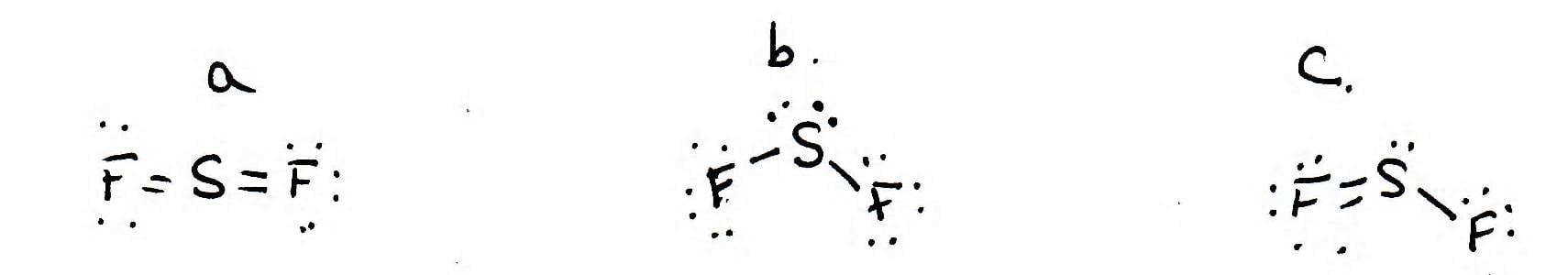







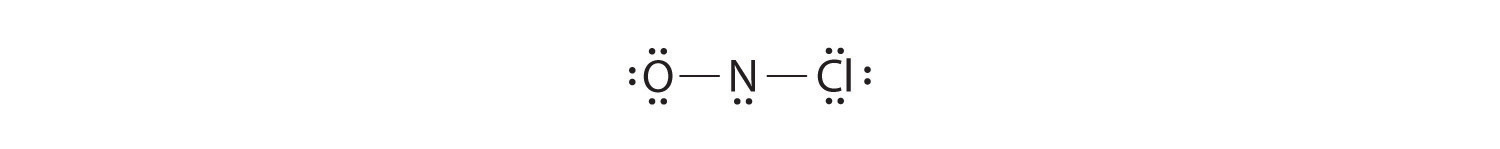




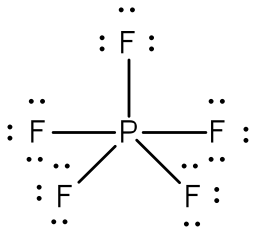
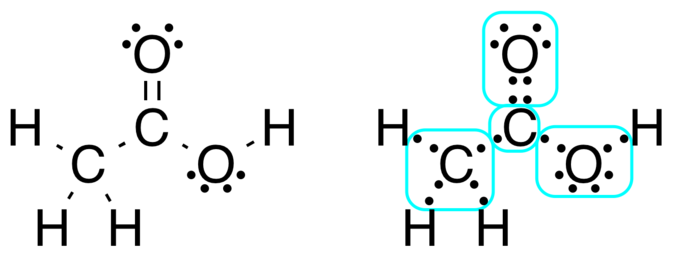

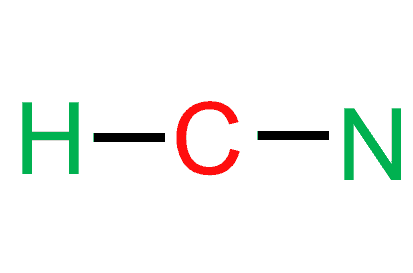



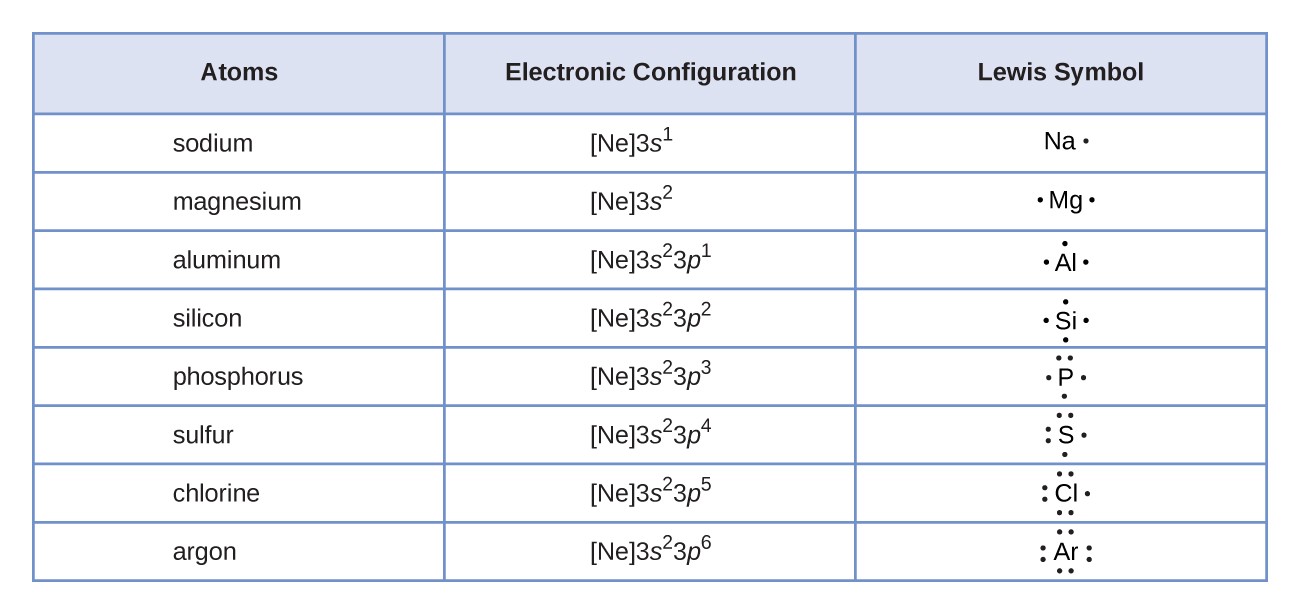
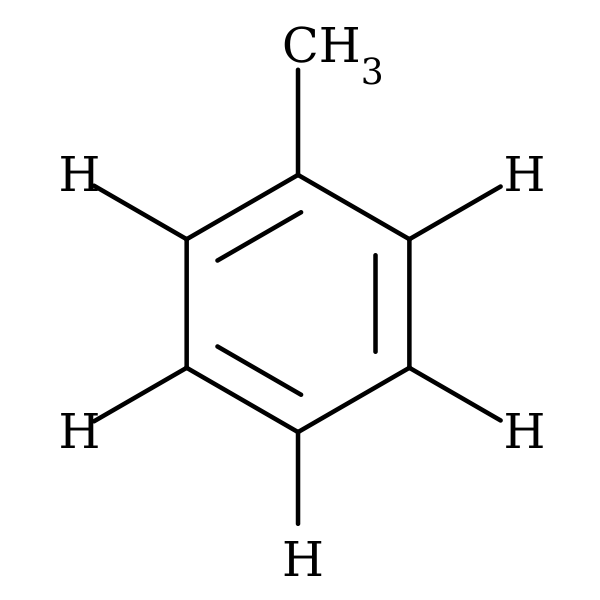



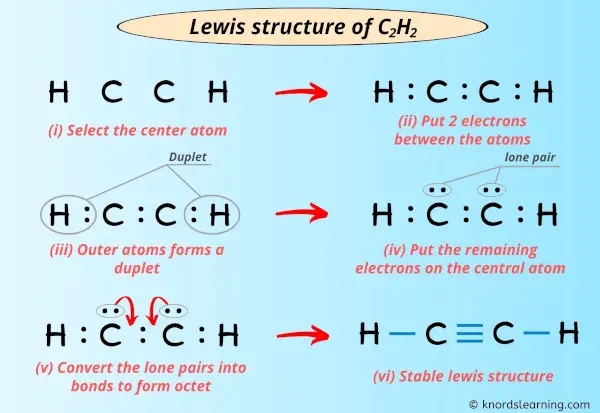
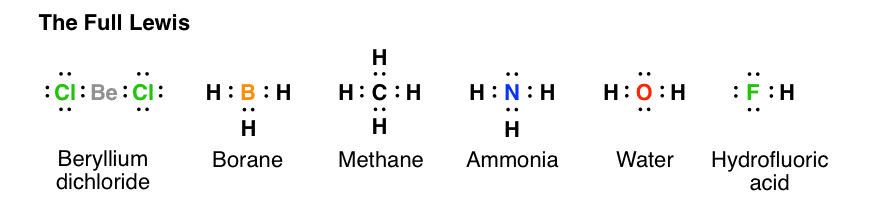


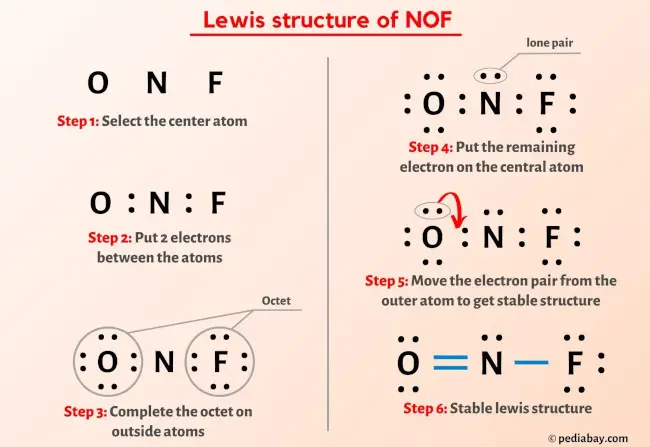

Post a Comment for "42 lewis symbols"